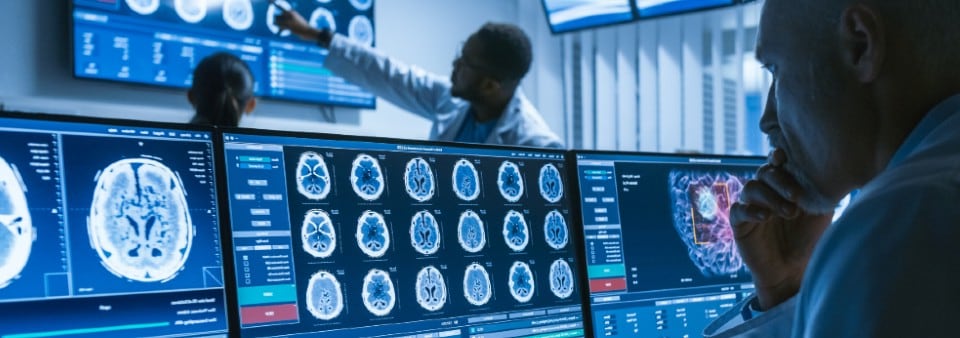
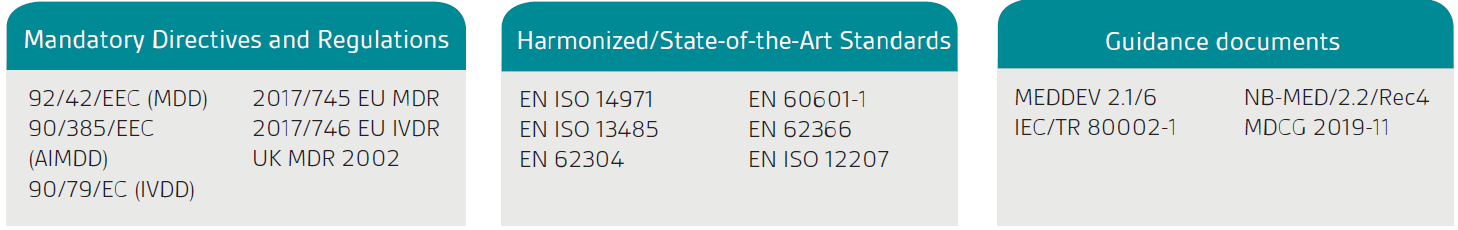
Placing your SaMD on the market.
SaMD and “Software in a Medical Device” (SiMD) are not defined in MDR, IVDR. MDCG 2019-11 defines “Medical Device Software” (MDSW) as encompassing all types of software with a medical purpose or acting as an accessory to a medical device, including software embedded within a dedicated hardware medical device.